Galvanic Corrosion - Some Observations From the Field
Part of any structural design process is selecting the material to build your structure from. This is an optimisation process, where you choose the materials based on their strength, ease of fabrication, durability, cost and other factors. Something that often gets overlooked in this process however is the potential for galvanic corrosion.
In mining, corrosive environments are common. Large amounts of water are used, and water is often contaminated with corrosive chemicals, and spillage of fine product holds moisture against steelwork (rather than letting it evaporate). Once mined, the product has to go somewhere, often to a port in a marine environment (plenty of salt!).
Therefore, an important design consideration is resistance to corrosion. Stainless steel would seem to be ideal to use in these locations. Unfortunately, stainless steel costs more than normal structural steel. Because of this, typically the following choices are made:
- Structural steel is used for large volume components which can be painted.
- Stainless steel is used for small items or items that aren’t possible to paint easily, such as:
- Services brackets are lightweight, thin, and often have complex profiles (low material cost combined with difficulty painting).
- Moving parts such as pins (paint fails at the interface with stationary parts).
- Items exposed to material flow / wear (paint would wear off).
- Anything that gets added to the structure after painting (e.g. site run services).
And then this happens:

What’s the Problem?
So what’s going on? Galvanic Corrosion - that’s what! But what is Galvanic Corrosion?
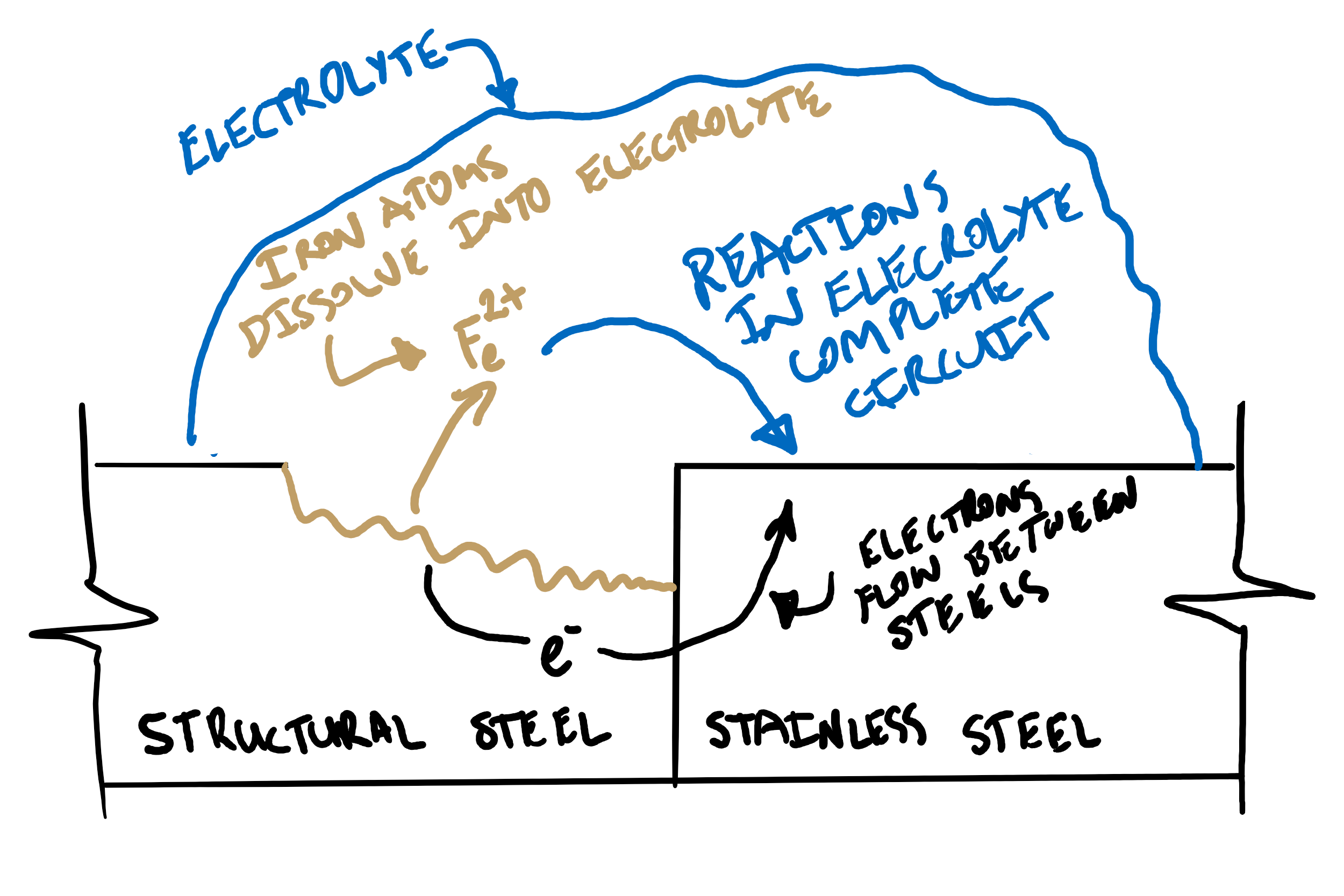
Corrosion is basically an electrochemical reaction where:
- One or more metals is in contact with a conductive solution called the Electrolyte (usually but not always water).
- At one location, some of the metal, known as the Anode, undergoes chemical Oxidation in which an oxidating chemical (usually but not always oxygen) in the electrolyte reacts with the anode. This causes the anode to give up some of its electrons, and dissolve into the electrolyte.
- These electrons travel to another region in the metal, known as the Cathode, via an electrical connection (usually direct physical contact).
- The extra electrons allow the cathode to chemically Reduce molecules in the electrolyte (it transfers some of the extra electrons to the electrolyte).
- As this is an electrical process, the circuit between the oxidising and reducing regions of the electrolyte needs to be completed to allow an electric current to flow. This occurs through further electrochemical reactions in the electrolyte.[1] [2] [3] [4]
If both the anode and the cathode are the same material (e.g. normal corrosion of steel) different areas on the surface of the metal act as the anode or cathode. Which is which depends on local surface conditions, chemical concentrations, temperature and other factors.
However, where two different metals are in contact, they will have different electrical potentials. Therefore there will already be a voltage difference between them, trying to drive electrons from one to the other, even before the corrosion reaction starts. Once a corrosion reaction starts, this potential difference allows one of the metals to act entirely as the anode, and the other to act entirely as the cathode.
This results in the metal which is the cathode being protected against corrosion. However, for the metal which is the anode, corrosion can be very rapid because the different electrical potential between the anode and cathode acts to increase the electric current between them, increasing the rate of chemical reactions and therefore the rate of corrosion. This is known as Galvanic Corrosion, or sometimes Dissimilar Metal Corrosion.[5] [6]
So knowing this, what happens when stainless steel and structural steel are placed in contact?
- The stainless steel, which is more cathodic than structural steel, becomes the cathode and attracts electrons away from the structural steel. Because it is the cathode, it is protected from further corrosion.
- In the structural steel becomes the anode, positively charged iron ions react with the electrolyte (usually water) and turn into various iron oxide molecules. Iron oxide is not able to remain bonded to the structural steel and either dissolves into the electrolyte, or sticks to the surface as rust.[7]
How Can We Avoid It?
Based on the corrosion process above, there are 3x conditions required for galvanic corrosion to occur:
- A pair of materials that have different electrical potentials.
- A direct electrical connection between them.
- An indirect electrolytic connection between them.
This suggests some ways of eliminating galvanic corrosion.
- Prevent the electrolytic connection.
- Prevent the direct electrical connection.
- Eliminate the different materials.
Prevent the Electrolytic Connection
The corrosion process needs an electrolyte, so eliminating the electrolyte can stop the process. This is effectively impossible to achieve in mine and port facilities, which use large quantities of water as process water and for washdown. Port facilities are near the ocean where saltwater spray from the ocean provides a constant supply of moisture.
However, good detailing of structural steel to minimise any areas that can trap moisture or spillage can help reduce the rate of galvanic corrosion by allowing wet areas to dry out. It won’t eliminate galvanic corrosion, but good detailing is always worthwhile, even when you’re not worried about galvanic corrosion.
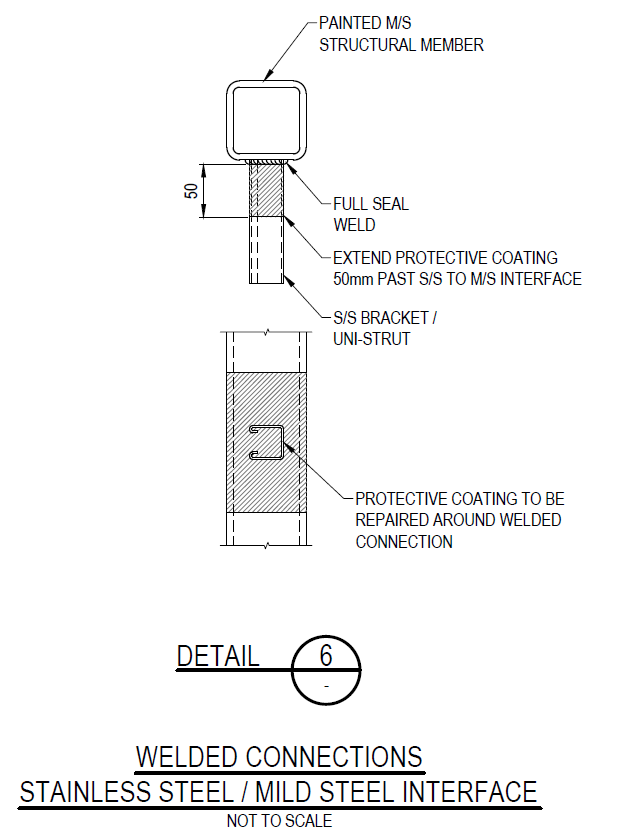
Painting the connection between materials can also eliminate the electrolytic connection. This attempts to prevent the electrolytic connection by placing a paint coating over the interface between stainless and structural steel. However, there are a number of problems with this approach:
- If the connection is a welded connection, any gas bubbles, undercut, over-reinforcement or areas of poor weld profile will be locations where the paint coating is too thin, too thick (prone to cracking) or simply doesn’t stick.
- If the connection is a bolted connection, the paint is likely to be too thick or thin over the joint line, and any movement between the parts will crack the paint.
- If the joint is a field joint, it is unlikely that a quality paint coating can be achieved due to dust, humidity, time constraints etc.
- One of the main reasons for using stainless steel is to avoid painting it, so the paint coating is usually only extended a nominal distance onto the stainless steel. However, some environments are wet enough that you can have electrolytic connections over even wide paint coats on the interface.
A paint coating is better than nothing, but in my experience don’t expect it to stop galvanic corrosion for long. The following figures show a typical detail and failure of this same detail within ≈5 years of installation:

Prevent the Direct Electrical Connection
The corrosion process also needs a direct electrical connection - preventing this connection will stop the corrosion. To eliminate the electrical connection:
- Avoid welded connections between structural and stainless steel.
- Bolted connections need to be isolated with insulating washers, bushes & insulating tape.
- Contact points (under floormesh / floorplate) should be isolated with isolating tapes, coatings etc.
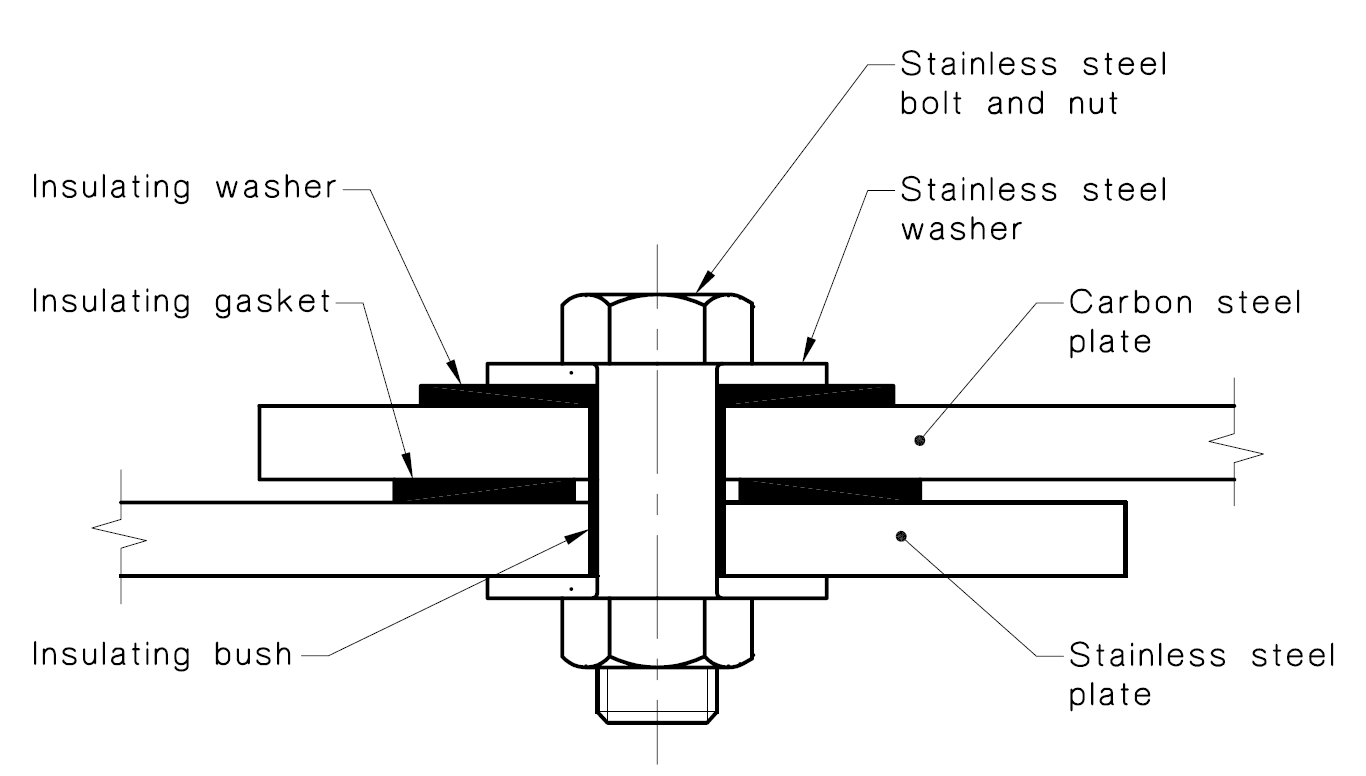 Image from Standards Australia, AS4673-2001 Cold-Formed Stainless Steel Structures, Figure C5
Image from Standards Australia, AS4673-2001 Cold-Formed Stainless Steel Structures, Figure C5
If not properly isolated, bolted connections can cause significant local corrosion damage. Care also needs to be taken when installing the isolating materials. Usually these are nylon washers and thin layers of insulating tape/rubber, which can be easily damaged by over-torquing or sharp edges. The following image shows an example where the nylon washers have been cracked by over-tightening (although corrosion to date appears to be caused by paint failure, not galvanic corrosion).

Use Only One Material
The best way to prevent galvanic corrosion is to use only one material, either structural steel or stainless steel. This eliminates the electrical potential difference that causes galvanic corrosion, and avoids any concern about damaging paint coatings or forgetting to install isolating materials. If the material you choose to use is structural steel, general corrosion can still occur but it should be less aggressive than galvanic corrosion would have been.
Use a Large Anode & a Small Cathode
While not on the list of 3 methods above, another recommendation sometimes given is to make sure that the area of the cathode is significantly smaller than the anode. The galvanic current produced during galvanic corrosion is related to the relative surface areas of the anode and the cathode. With a small cathode and a large anode, the galvanic current should be low.
This is used to justify the use of stainless steel fasteners (bolts, screws etc.) even without isolation. The stainless steel fastener (cathode) has a small surface area compared to the rest of the structure (the anode) so galvanic corrosion rates should be low. However, in my experience of inspecting structures, this is often ineffective at preventing corrosion.
This is because bare structural steel is almost always painted to avoid general atmospheric corrosion, regardless of the presence or absence of stainless steel fittings. Paint is never 100% effective, but early in its life it is usually very good at isolating the structural steel from any potential electrolytes. However, near the fasteners, there is a narrow ring of paint that has been damaged by the fastener itself, or the tools used to tighten them.
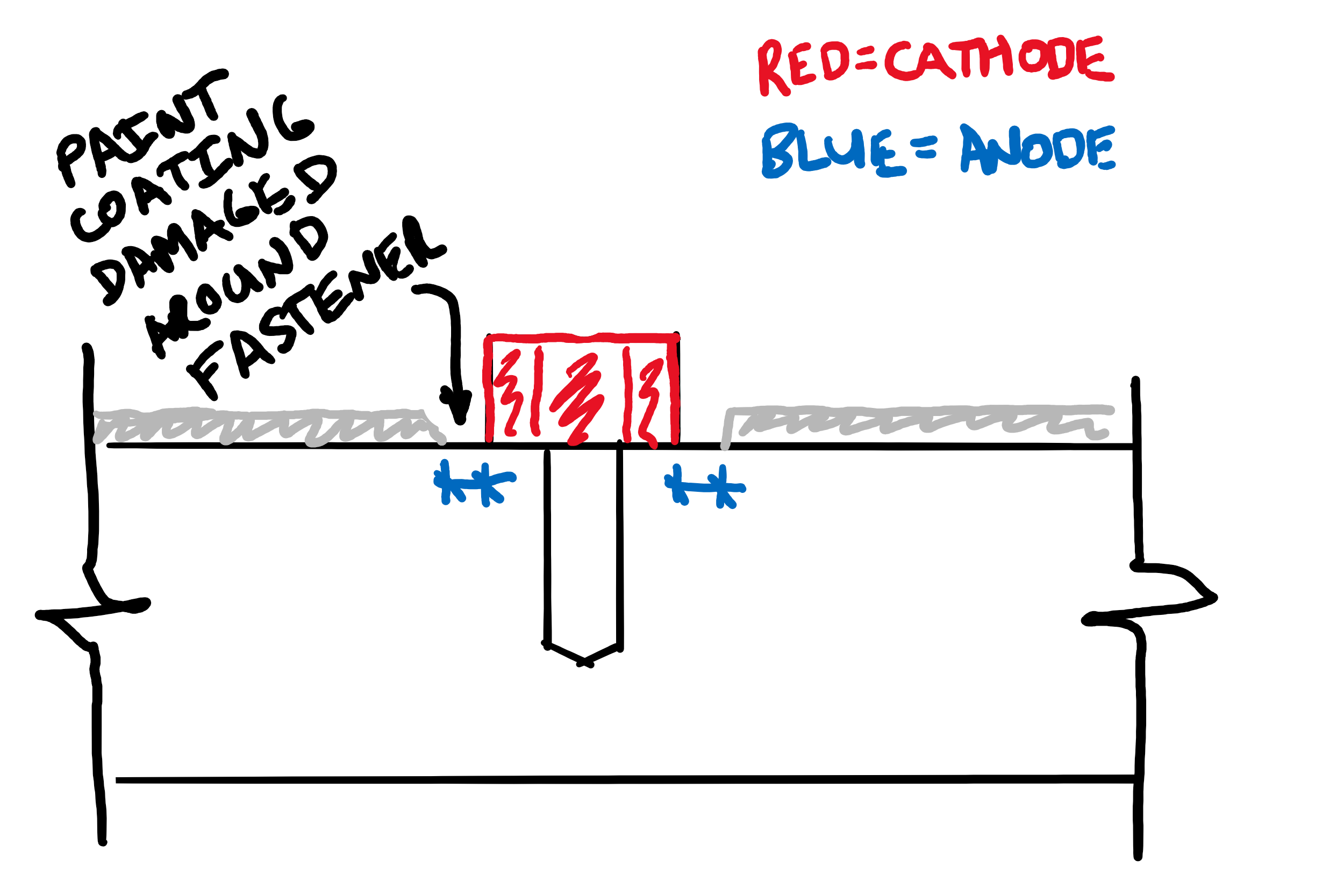
The actual relative areas that need to be considered are NOT the entire structural steel structure compared to the stainless fasteners. Instead, they are the very small area of anode in the ring around the fastener and the (relatively) large surface area of the fastener head. This is actually a large cathode, small anode situation, and can result in very aggressive local corrosion as the following photo I took a few weeks ago shows:

You might be thinking to yourself though, “That’s obviously a small bolt for a services attachment, who cares?”. However, that’s only the most recent photo I had easily available. Stair treads and grating clips are common sources of galvanic corrosion that I see, and failure of a stair tread or a mesh panel could easily be fatal (see this post).
Even more concerning is the use of unprotected and un-isolated stainless steel pins in major structures. In this case, galvanic corrosion combines with movement of the joint, which works any rust products free and ensures that good metal-to-metal contact, resulting in extremely rapid corrosion. The following figures show the corrosion damage that occurred within ≈5 years of installation of stainless steel pins into a mild steel lug. These pins supported an operator’s cabin, and failure of this connection could have resulted in the death of the operator.


So When Can I Use Stainless Steel with My Structural Steel?
It’s not always a bad idea to combine stainless and structural steels, if done properly. From what I’ve seen on site though, the following rules should be followed:
- Stainless steel doesn’t excuse bad detailing.
- Always detail your structures so they don’t catch water or spillage and can drain.
- Design structures and especially connections that can be inspected, maintained and replaced, so that when galvanic corrosion or any other damage occurs it can be found and fixed.
- For pins and any other items that can move, don’t combine stainless and structural steel. Any movement in the joint will damage any isolation you tried to put into place and allow corrosion to occur.
- If you must use a stainless pin, use stainless lugs & clevises, and bolt them on as a unit.
- Avoid welding stainless steel to structural steel. This is almost always a bad idea.
- Consider using sacrificial structural steel bosses / cleats welded to the structural steel, with holes drilled / tapped for stainless fasteners.
- If welding stainless steel to structural steel can’t be avoided, use high quality welds and grind them smooth to take a good quality paint coating. Extend the paint coating as far from the joint as you can. If at all possible, do the welds and the paint coat in a workshop, not on site, for better coating quality.
- Ensure that all bolted joints are properly isolated.
- Don’t forget the bolt shanks inside the hole, and the faying surfaces between clamped parts.
- If at all possible, paint the stainless bolt / fitting to repair any paint damaged during installation.
- The “Small Cathode, Large Anode” effect may work in your favour, but don’t trust it unless you can ensure the cathode really is small compared to the real anode.
- In my experience, a large proportion of improperly installed stainless steel items are not structural. Owners & operators of mine and port facilities need to educate all their tradespeople including electricians, plumbers, carpenters and other trades on what is acceptable and what isn’t when it comes to installing services on existing structures.